Easily manage the entire lifecycle of IRB protocols
Your organization’s integrity depends on ensuring research is ethical. But that can be difficult when protocol processes are lengthy and convoluted. And if your systems are manual or outdated, that limits visibility into protocol status even more.
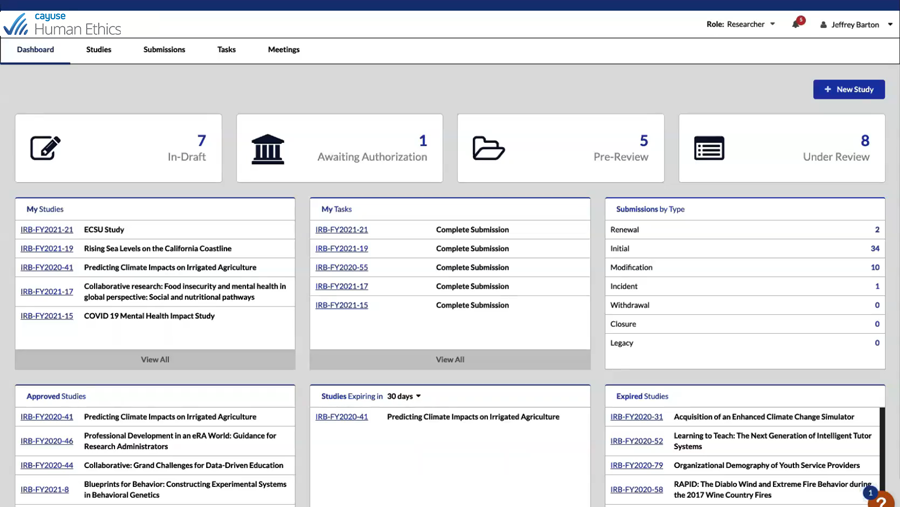
Get transparency into all phases of a protocol with Human Ethics. Enjoy increased efficiency with 100% electronic preparation, submission, and routing of human research study protocols. It’s never been easier for administrators, researchers, and committee members to collaborate.
Faster, more compliant human protocol management
Human Ethics integrates with your existing systems, and reporting CITI® training is easy
Accelerate review process
Improve transparency and collaboration
Reduce noncompliance risk

Smart forms for faster protocol development
Make researchers happy by saving them valuable time developing protocols with configurable smart forms:
- Familiar form templates developed with an AAHRPP-accredited partner
- Intuitive drag-and-drop builder
- Ability to revert to previous drafts
- Skip logic or conditional branching allowing for answering questions relevant to the study

Powerful features
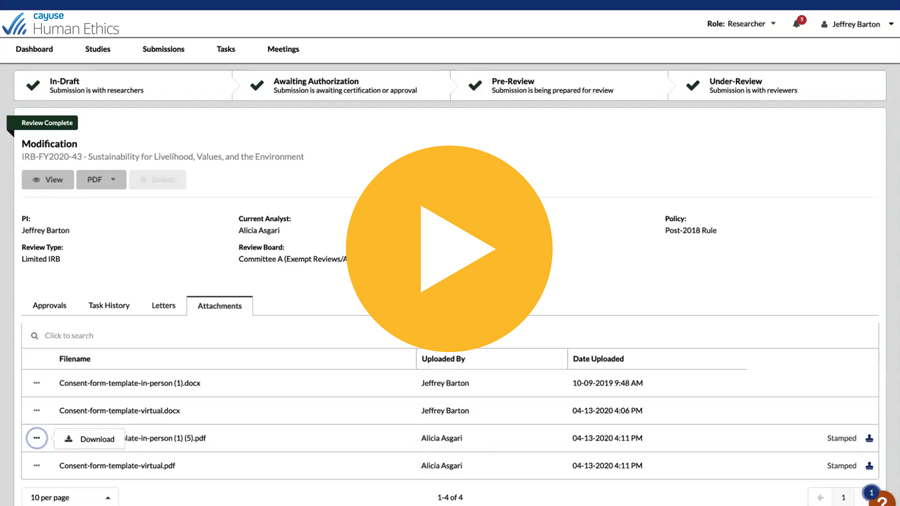
Collaborate online for submission, review, and approval with electronic routing, system alerts, and notifications
Compare submissions side by side for faster protocol review
See activity pipeline and protocol status with role-based dashboards and automatic reminders
Manage meetings with increased efficiency and compliance
“The Human Ethics dashboard is fantastic. The review trail allows us to see if something is hung up and where. In the past, it was unheard of to turn around something in 48 hours. Now we can do that. It’s really that much of a game-changer.”
Jonathan Lyon
Vice Provost

Request a video overview
Human Ethics
Improve collaboration among administrators, researchers, and committee members with electronic protocol preparation, submission, and routing.
Animal Oversight
Increase transparency of animal research protocols. Shorten turnaround time for protocol review and approval while reducing noncompliance risks.
Hazard Safety
Stay compliant, reduce approval time, and remove complexity from the protocol process. Keep research on schedule with improved collaboration and transparency.
Outside Interests
Make the disclosure process painless and increase faculty participation. Complete, track, and manage disclosures in minutes for more efficient research administration.
Over 700 top global research organizations trust Cayuse