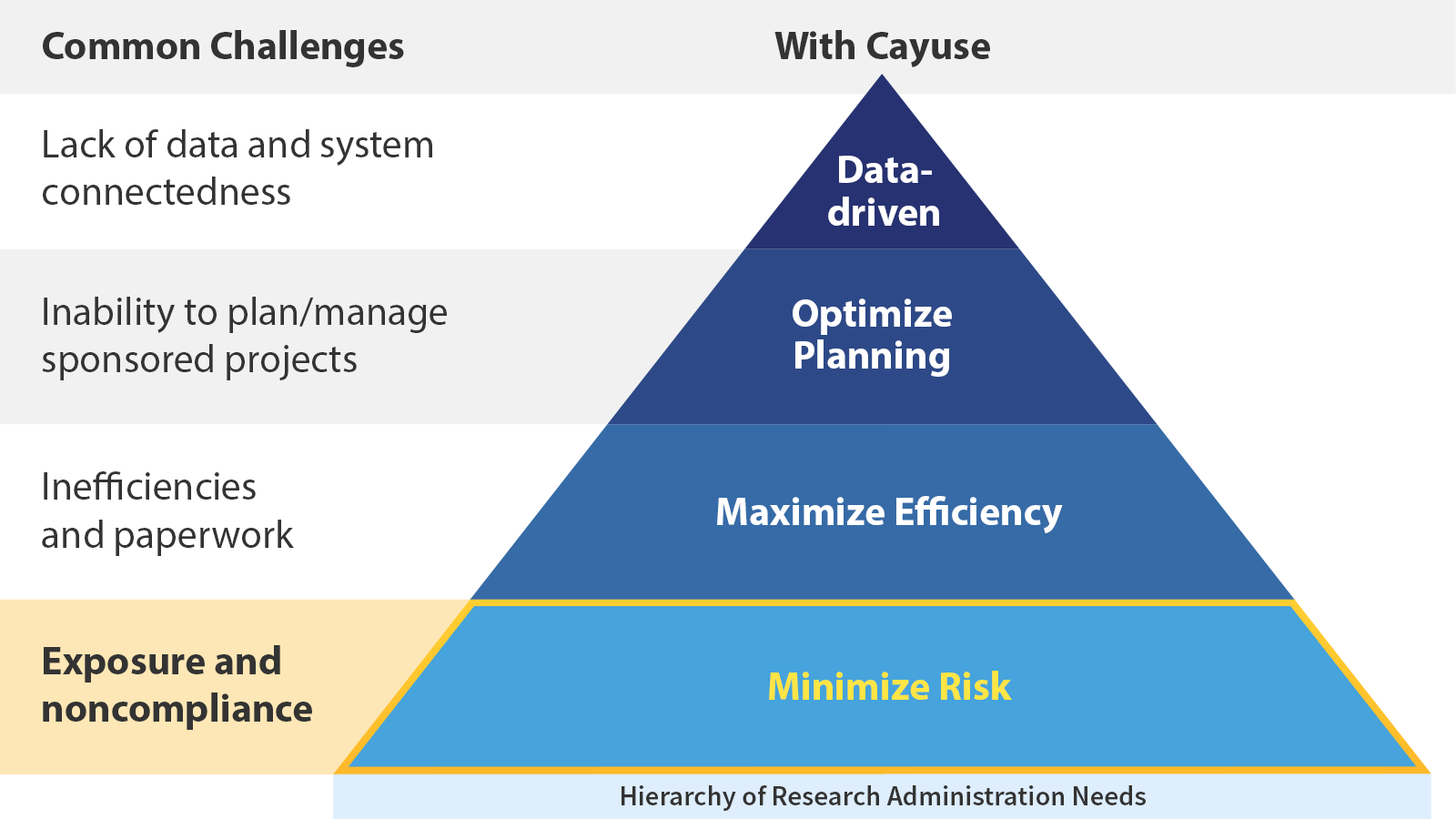
Minimize risk all in one place
“Cayuse has completely enabled us to be ahead of regulatory compliance. We’re proactive instead of reactive.”
Lorraine Bell
Training Program Manager

Why is risk a foundational challenge?
From fines and lost funding to negative press and harm to researchers, the consequences of noncompliance are serious. Too much is at stake to not worry about compliance–which is where the right partner comes in.
Cayuse helps in several ways:
Data Integrity & Automation
Create an audit trail and eliminate manual data entry, resulting in fewer errors.
Reviews
Increase transparency into protocols and their statuses for more informed reviews.
Approvals
Track in a centralized easy-to-find place–no more trying to find a piece of paper.
Protocol Routing
Keep things moving from submission and review to approval with notifications when action is needed.
“The compliance dashboard is fantastic. The review trail allows us to see if something is hung up and where. In the past, it was unheard of to turn around something in 48 hours. Now we can do that. It’s really that much of a game-changer.”
Jonathan Lyon
Vice Provost
Manage risk in every area
Human Ethics
Efficiently prepare, submit, and route IRB protocols for review.
Hazard Safety
Easily submit IBC protocols for approval before research starts.
Animal Oversight
Submit IACUC protocols and review meetings more efficiently.
Outside Interests
Maintain high ethical standards by simplifying COI disclosures.
Over 700 top global research organizations trust Cayuse