Creating and managing IBC protocols doesn’t have to be a hassle
Research with biological materials and other potentially hazardous agents is highly regulated to protect laboratory staff, the public, and the environment. But ensuring that protocols adhere to IBC and NIH requirements can be time-consuming.
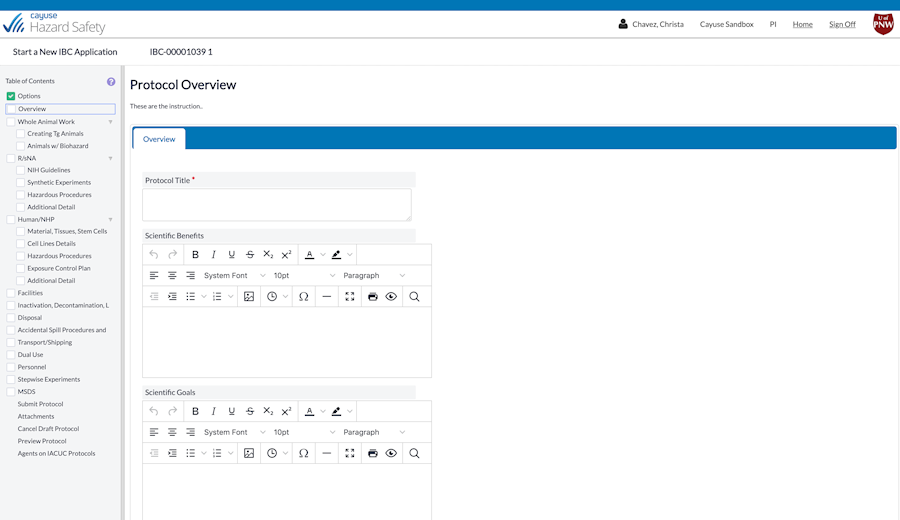
What if you could stay safe and remain in compliance without sacrificing efficiency? It’s possible with Hazard Safety. Now you can quickly and easily manage a protocol’s entire lifecycle, from authorship to end of study.
Faster IBC protocol management
Hazard Safety is 100% paperless and integrates with your existing systems
Shorten approval time
Improve internal collaboration
Reduce the risk of noncompliance
Improved compliance
Ensure research efforts conform to biosafety guidelines with built-in industry components:
- Workflows for compliance and protocols
- Agenda management
- Committee meeting management
- Document reviews
- Automatic notifications for continuations and De Novo reviews

Powerful features
Improve internal collaboration and shorten protocol approval time with fully built-in workflow
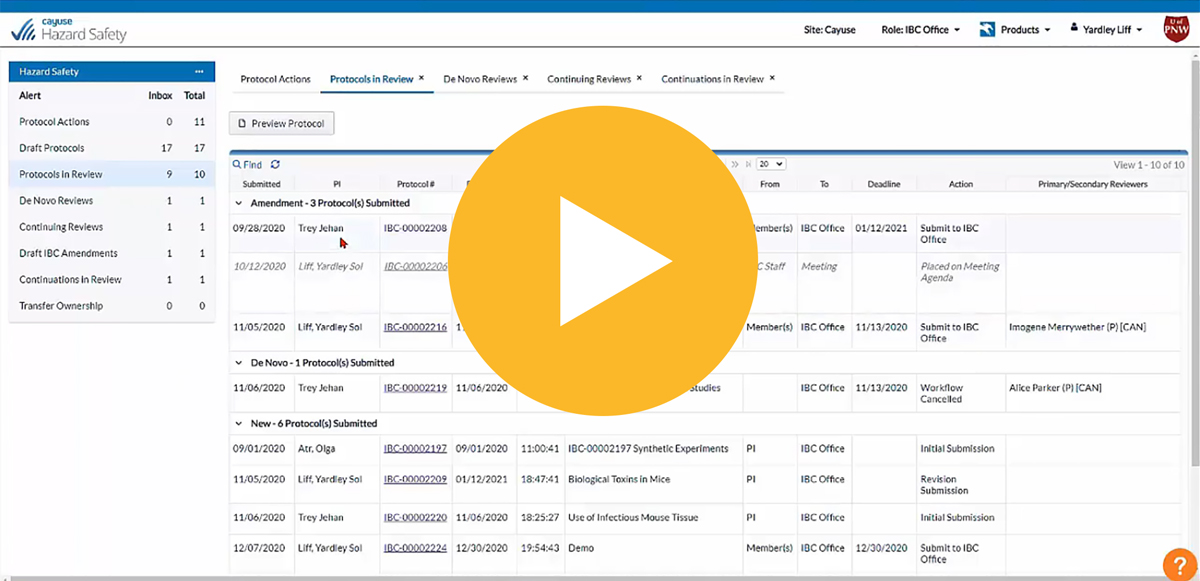
Review protocols more quickly with access to the latest version, track changes, and side-by-side version comparison
Know the protocol status at all times with homepage dashboards displaying all critical workflow information
“We were looking for cloud-based IBC and IACUC solutions that would easily share data with each other. We evaluated five vendors, and when we saw Hazard Safety, it literally made us smile. It’s clear that Cayuse knows what compliance offices need for success.”
Louise Griffin
Senior Director of Research and Sponsored Programs Administration

Request a video overview
Human Ethics
Improve collaboration among administrators, researchers, and committee members with electronic protocol preparation, submission, and routing.
Animal Oversight
Increase transparency of animal research protocols. Shorten turnaround time for protocol review and approval while reducing noncompliance risks.
Hazard Safety
Stay compliant, reduce approval time, and remove complexity from the protocol process. Keep research on schedule with improved collaboration and transparency.
Outside Interests
Make the disclosure process painless and increase faculty participation. Complete, track, and manage disclosures in minutes for more efficient research administration.
Over 700 top global research organizations trust Cayuse

























Uni of Northern Iowa