If your IACUC processes could be more efficient (and whose couldn’t?), three experts have some proven tips for you. We’ve summarized some of the great advice from Chapman University’s Bruce Kennedy, Biogen’s Cheryl Cheney, and UC Denver’s Laura Richardson from their recent Connect Conference session below, along with a recording of their full session:
Keep reading for some of the highlights shared during this informative session!
Use automated email notifications
Bruce Kennedy, IACUC Administrator at Chapman University
It’s been scientifically proven that your brain can only keep so much information in the foreground (see: remembering Taylor Swift lyrics but forgetting why you went into a room). So thank goodness we have notifications on our phones and calendars to remind us of upcoming meetings and deadlines.
That was what Bruce Kennedy liked about Cayuse’s Human Ethics software 12 years ago (formerly called Cayuse IRB). He found it was really helpful to get notification emails when IRB protocols would progress to the next step or needed approval. Fast forward to today, when he works in human and animal research administration at Chapman University. He wanted to implement something like Cayuse IRB for animal research protocols, so in addition to the modernization efforts across the entire research lifecycle that Chapman has been partnering with Cayuse on for several years, Cayuse’s Animal Oversight app was another addition to help increase productivity while minimizing risk for Chapman’s research teams.
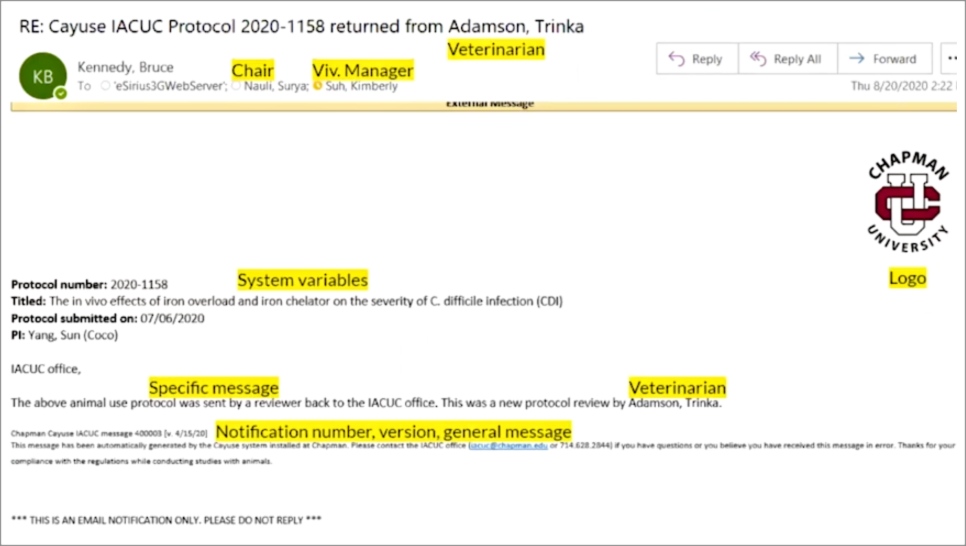
“I learned that I could easily set up email alerts to specific people and teams, and Odessa [my Cayuse Professional Services Consultant] helped show me just how easy it was,” he says. He configured an automatic email notification that the Cayuse system at Chapman sends to the department chair, vivarium manager, and veterinarian. Bruce built the automated email just how he wanted it (in this case, a return of a review from the veterinarian). “The message can have specifics that are useful for the PI, IACUC office, or whomever the recipient,” he says. Before any of these emails went out, Bruce alerted PIs, IACUC members, and others that they would be getting email notifications from the Cayuse system so they weren’t surprised.
Here’s exactly what Bruce configured to appear in this particular email:
- Chapman University logo
- Protocol number
- Title of protocol
- When the protocol was submitted
- Who the investigator is
- Notification number and version
- General message
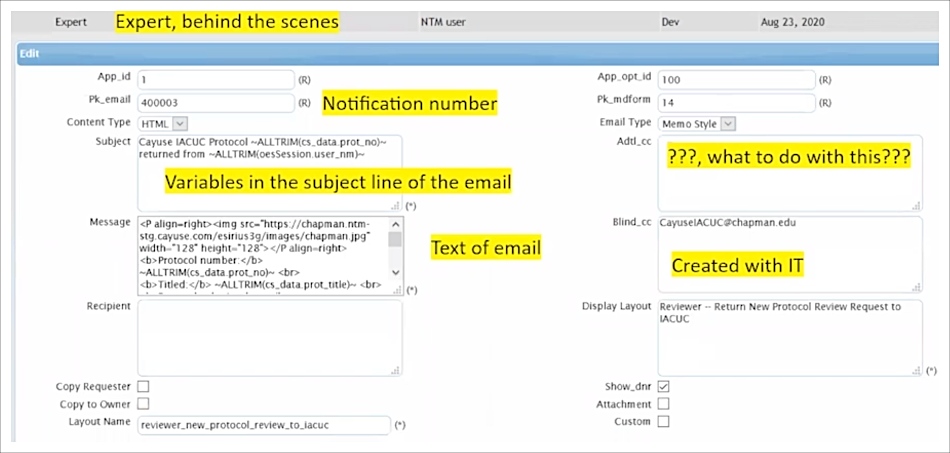
With Cayuse’s Expert tool, Bruce can even create or configure the HTML code behind the scenes. During the implementation process with Cayuse, Bruce added other variables to the email builder, including automatically blind copying (BCCing) all notification emails to an archive email account that Bruce can search if necessary. It’s not required, but the Expert tool gives you the freedom to incorporate formatting, variables, and URLs if desired.
Set up veterinary verification and consultation (VVC) reviews
Cheryl Cheney, IACUC Coordinator at Biogen
Typically, changes to an IACUC protocol have to be re-reviewed by your research organization’s IACUC committee, which can take a while if the process is completely paper-based. But in August 2014, the National Institutes of Health (NIH) issued a notice that makes this process a little faster and easier. NOT-OD-14-126 says that if an IACUC protocol is only changing in certain minimal ways, veterinarians can verify that the changes satisfy IACUC policies and guidelines and can be approved.
It’s important to note that veterinarians can only review and approve protocol amendments, not brand-new protocols. Here’s the breakdown of what they can and can’t approve:
| Changes vets can approve | Changes vets cannot approve |
|
|
For example, if the researcher will be taking an animal blood sample from one type of vein and wants to switch to another vein, a vet could approve that change (or formally speaking, that amendment is eligible for VVC review). But if taking a blood sample wasn’t part of the original protocol at all, that change would have to go back to the IACUC committee.
Several features in Cayuse’s Animal Oversight app can help with VVC review:
- Reason for amendment fields
- Protocol introduction
- Table of contents
- Preview protocols (and compare to previous version)
- Full document review
To set up veterinarians in Animal Oversight, add them to Contact Management and Site Management (assign the Staff Vet role) and choose the process: either submitting the amendment to one vet or multiple members. Once you assign the amendment to the veterinarian, s/he can double-check the PI’s revisions, enter notes and documentation, and the changes can be added to minutes for a future IACUC committee meeting. VVC isn’t for everyone, but if you use Cayuse Animal Oversight, it can definitely help streamline certain changes to protocols.
Integrate IACUC and IBC systems
Laura Richardson, IACUC, University of Colorado – Denver
There are a few different ways you can ensure that animal research protocols involving IBC agents are approved by both your IBC and IACUC committees: the protocols could be linked in your database, your committee coordinators have established a good system for communication, or another way. But some organizations have no way to ensure that at all!
Using Cayuse’s Animal Oversight (formerly IACUC) and Hazard Safety (formerly IBC), Laura Richardson and her team have an IBC form linked to the IACUC form through the PI’s name. If the PI starts an IBC protocol and indicates there that they have IBC agents going into live animals, those agents are automatically moved to a spot in the system where they can be added to the IACUC protocol:
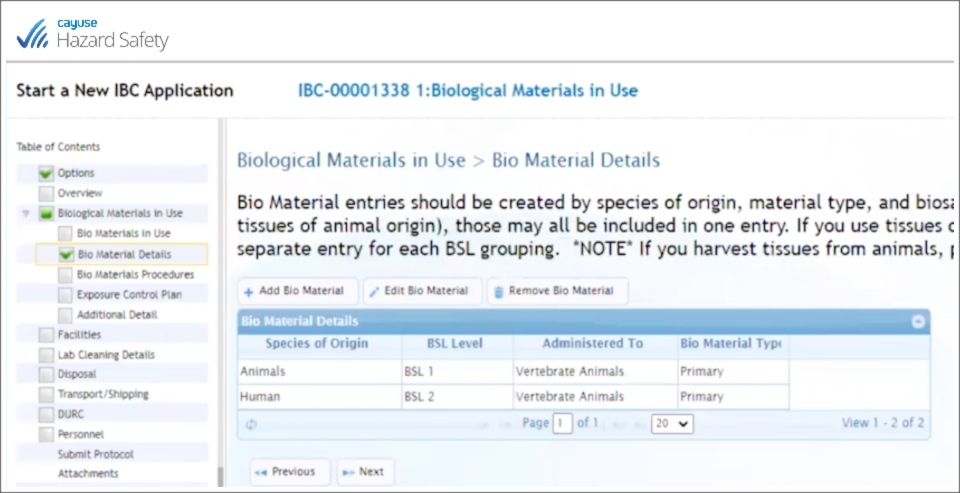
Cayuse’s system passes the IBC agents to the IACUC form, and within Animal Oversight, you can dig into the details of the IBC agent and access protocol agent information. You save time and stay in compliance—everybody wins.
Huge thanks again to Chapman University’s Bruce Kennedy, Biogen’s Cheryl Cheney, and UC Denver’s Laura Richardson for sharing their knowledge, best practices, and tips for leveraging technology to optimally support research teams while ensuring IACUC, IBC, IRB compliance.
To learn more about making your animal oversight practices more efficient, check out Cayuse Hazard Safety and Animal Oversight, or get in touch today!


