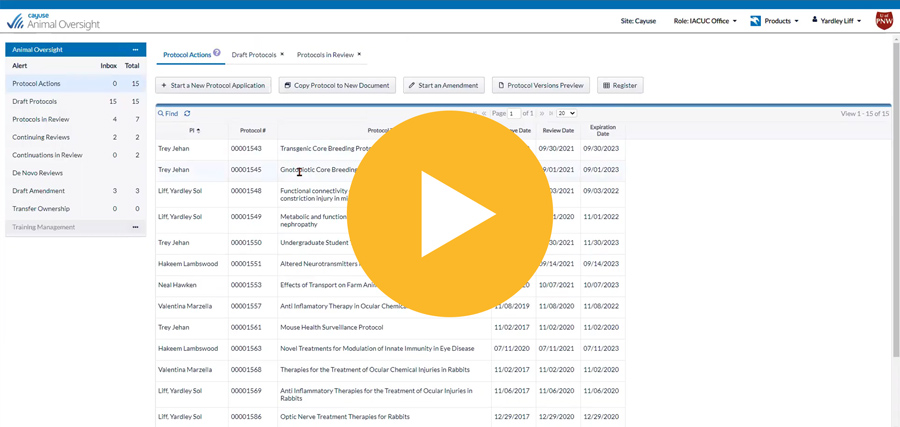
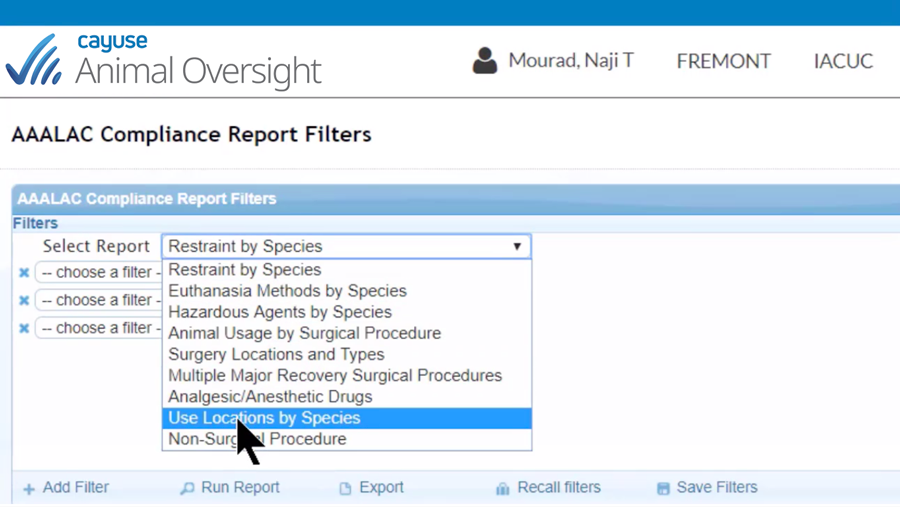
Easily manage the entire lifecycle of IACUC protocols
Compliance with animal research regulations is essential, but it’s taxing on your organization’s entire research community. Protocol review and approval is a major undertaking. Without transparency and efficient collaboration, it’s time-consuming and can delay research start time.
Are you ready to save time, increase transparency, and make researchers and staff happier? It’s possible with Animal Oversight, which helps you electronically prepare, submit, and route studies for committee approval.
Faster animal protocol management
Animal Oversight is 100% paperless and integrates with your existing systems
Shorten turnaround time
Improve internal communication
Reduce the risk of noncompliance
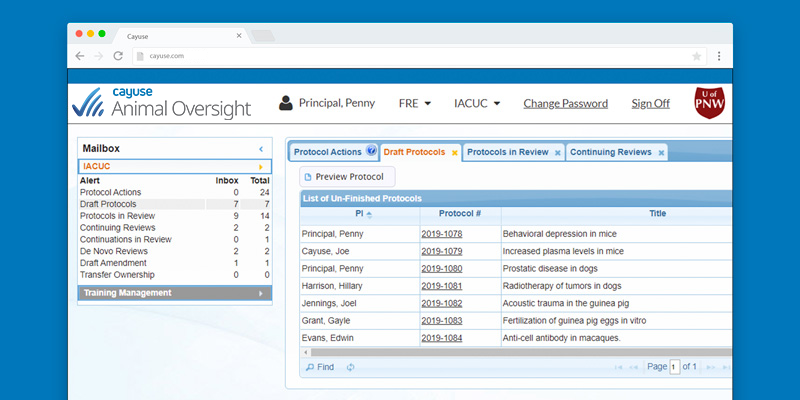
Improved compliance
Ensure research efforts conform to ethical guidelines with built-in industry components:
- Workflows for compliance and protocols
- Agenda management
- Committee meeting management
- Document reviews
- Automatic notifications for annual and De Novo renewals
- Filter-driven USDA and AAALAC reports
- Training status monitoring and management
- Configurable training requirements for activities, species, procedures, and hazardous agents
- Transparency into personnel qualifications during protocol authorship

Powerful features
Improve internal collaboration and shorten protocol approval time with fully built-in workflow
Review protocols faster with instantaneous access to the latest version, track changes, and side-by-side version comparison
Know protocol status at all times with homepage dashboards displaying all critical workflow information
Support multiple independent IACUCs within the same organization
Automatically track training requirements at the personnel, species, and activity level with Training Records
“We have been pleasantly surprised by how quick and easy the program is to use. Some researchers were submitting protocols without any training.”
Mark Douse, Ph.D., Director
Office for Research Committee Support

Request a video overview
Human Ethics
Improve collaboration among administrators, researchers, and committee members with electronic protocol preparation, submission, and routing.
Animal Oversight
Increase transparency of animal research protocols. Shorten turnaround time for protocol review and approval while reducing noncompliance risks.
Hazard Safety
Stay compliant, reduce approval time, and remove complexity from the protocol process. Keep research on schedule with improved collaboration and transparency.
Outside Interests
Make the disclosure process painless and increase faculty participation. Complete, track, and manage disclosures in minutes for more efficient research administration.
Over 700 top global research organizations trust Cayuse