Animal research training records management software
Keep researchers and animals safe
Make sure staff have the training they need
Before research facility staff can work with animals or biohazards, they need proper training—for compliance as well as peace of mind. But when you have lots of protocols and staff members, keeping track of everyone’s qualifications and matching them with research activities is a monumental task.
It’s easy with Training Records. This intuitive solution is the best way to track staff certifications, improve compliance, and ensure everyone’s safety.
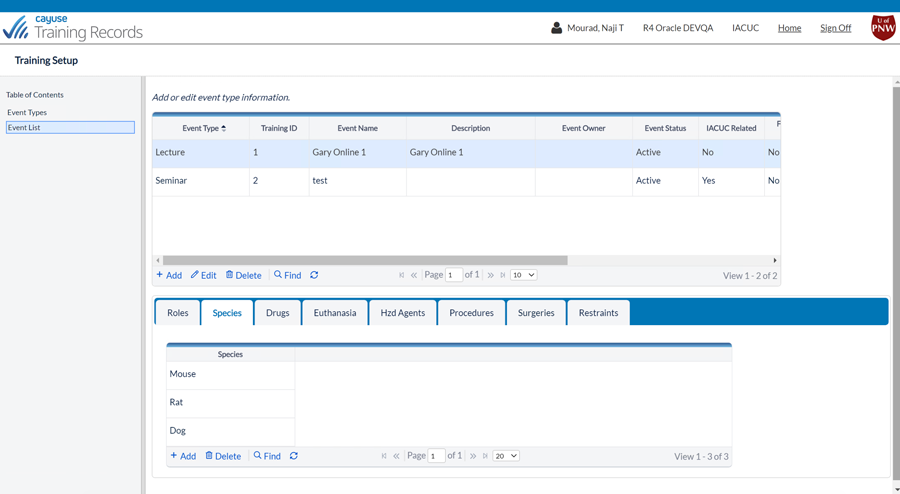
Better training management
Training Records is 100% paperless and integrates with your existing systems
Save time
Improve staff safety
Reduce risk of noncompliance
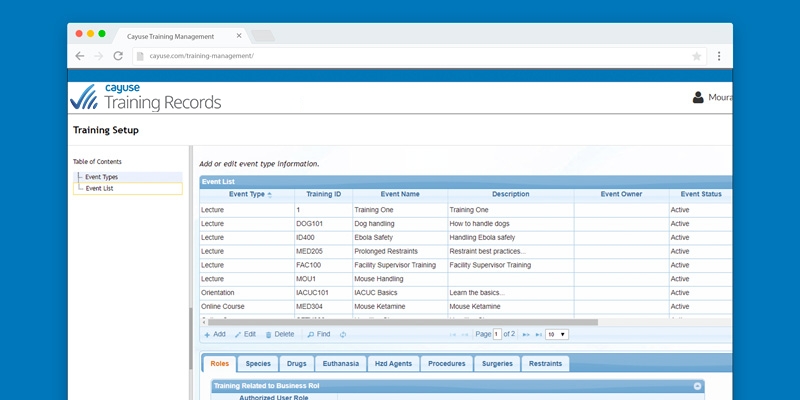
Improved compliance
With Training Records, researchers can easily view personnel qualifications during protocol authorship to ensure staff listed on their protocol have or will receive the required training.
Training coordinators and management can also view personnel qualifications to ensure staff have received the required training for the research activities.

Powerful features
Set up requirements by activities, species, procedures, and hazardous agents
Track training at the personnel, species, and activity level
Expedite protocol authorship and approval by automatically tracking training requirements
Human Ethics
Improve collaboration among administrators, researchers, and committee members with electronic protocol preparation, submission, and routing.
Animal Oversight
Increase transparency of animal research protocols. Shorten turnaround time for protocol review and approval while reducing noncompliance risks.
Hazard Safety
Stay compliant, reduce approval time, and remove complexity from the protocol process. Keep research on schedule with improved collaboration and transparency.
Outside Interests
Make the disclosure process painless and increase faculty participation. Complete, track, and manage disclosures in minutes for more efficient research administration.
Training Records
Expedite the protocol approval process and foster a culture of safety by automatically cross-referencing staff qualifications with research activities.
Over 670 top global research organizations trust Cayuse






































Request a personalized demo
See why over 670 research organizations use Cayuse